We all need some space sometimes, right? That’s true down to a molecular level. Molecules don’t like to stay too close together and will try to move to less crowded areas. That process is called diffusion and we will explore all about it in this simple but revealing experiment.
Article Contents
1. What is Diffusion?2. What causes Diffusion?
3. Factors that influence Diffusion
4. Materials needed for demonstrating Diffusion
5. Instructions for demonstrating diffusion
6. What will you develop and learn
What is Diffusion?
Have you ever smelled your neighbor’s lunch on your way home? Or smelled someone’s perfume minutes after that person was gone? You experienced the diffusion!
Diffusion is a movement of particles from the area of high concentration to an area of low concentration. It usually occurs in liquids and gases.
Let’s get some complex-sounding terminology out of the way. When talking about diffusion, we often hear something about the concentration gradient (or electrical gradient if looking at electrons). Gradient just means a change in the quantity of a variable over some distance. In the case of concentration gradient, a variable that changes is the concentration of a substance. So we can define the concentration gradient as space over which the concentration of our substance changes.
For example, think of the situation when we spray the air freshener in the room. There is one spot where the concentration of our substance is very high (where we sprayed it initially) and in the rest of the room it is very low (nothing initially). Slowly concentration gradient is diffusing – our freshener is moving through the air. When the concentration gradient is diffused, we reach equilibrium – the state at which a substance is equally distributed throughout a space.
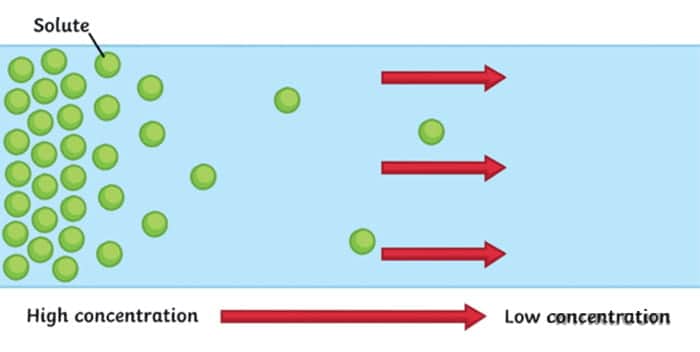
It’s important to note that particles never stop moving, even after the equilibrium is reached. Imagine two parts of the room divided by a line. It may seem like nothing is happening, but particles from both sides are moving back and forth. It’s just that it is an equal probability of them moving from left to right as it’s from right to left. So we can’t notice any net change.
Diffusion is a type of passive transport. That means it doesn’t require energy to start. It happens naturally, without any shaking or stirring.
There is also a facilitated diffusion which happens in the cell membranes when molecules are transported with the help of the proteins.
You may remember hearing about Osmosis and think about how is this different from it. It is actually a very similar concept. Osmosis is just a diffusion through the partially permeable membrane. We talked about it more in our Gummy Bear Osmosis Experiment so definitely check it out.
What causes Diffusion?
Do particles really want to move somewhere less crowded? Well, no, not in the way we would think of it. There is no planning around, just the probability.
All fluids are bound to the same physical laws – studied by Fluid mechanics, part of the physics. We usually think of fluids as liquids, but in fact, air and other types of gas are also fluids! By definition, fluid is a substance that has no fixed shape and yields easily to external pressure.
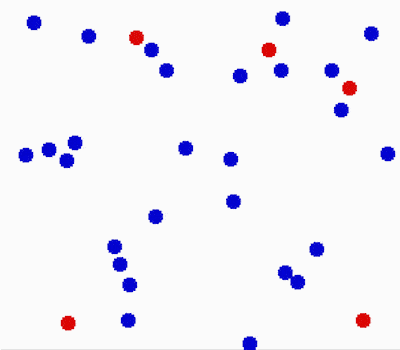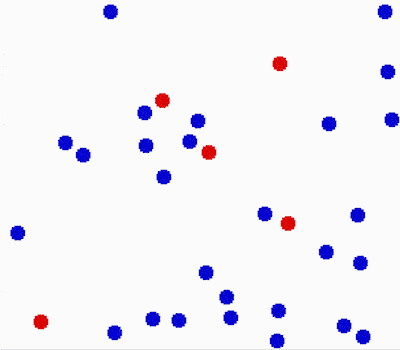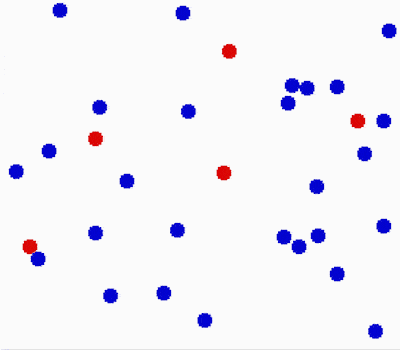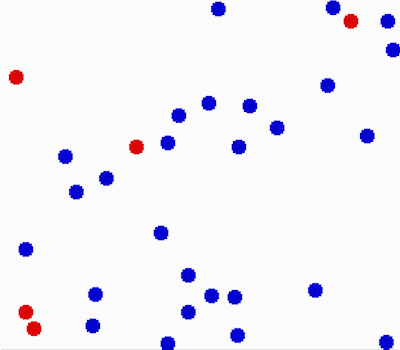
Another property of the fluids is that they flow or move around. Molecules in fluids move around randomly and that causes collisions between them and makes them bounce off in different directions.
This random motion of particles in a fluid is called Brownian motion. It was named by the biologist Robert Brown who observed and described the phenomenon in 1827. While doing some experiments with pollen under the microscope, he noticed it wiggles in the water. He concluded that pollen must be alive. Even though his theory was far off, his observation was important in proving the existence of atoms and molecules.
Factors that influence Diffusion
There are several factors that influence the speed of diffusion. The first is the extent of the concentration gradient. The bigger the difference in concentration over the gradient, the faster diffusion occurs.
Another important factor is the distance over which our particles are moving. We can look at it as the size of a container. As you may imagine, with the bigger distance, diffusion is slower, since particles need to move further.
Then we have characteristics of the solvent and substance. The most notable is the mass of the substance and density of the solvent. Heavier molecules move more slowly; therefore, they diffuse more slowly. And it’s a similar case with the density of the solvent. As density increases, the rate of diffusion decreases. It’s harder to move through the denser solvent, therefore our molecules slow down.
And the last factor we will discuss is the temperature. Both heating and cooling change the kinetic energy of the particles in our substance. In the case of heating, we are increasing the kinetic energy of our particles and that makes them move a lot quicker. So the higher the temperature, the higher the diffusion rate.
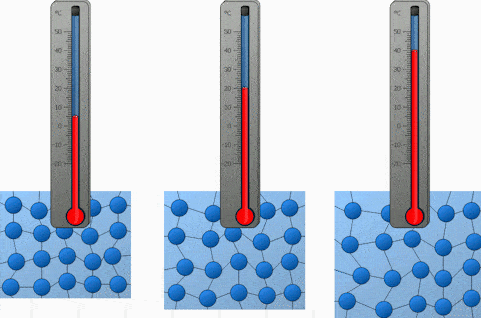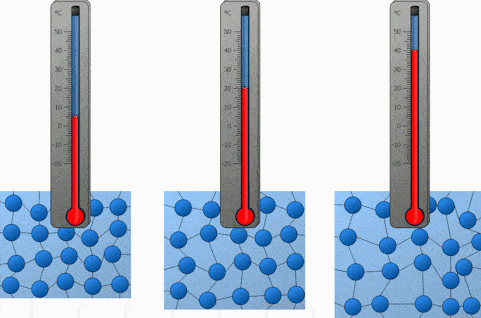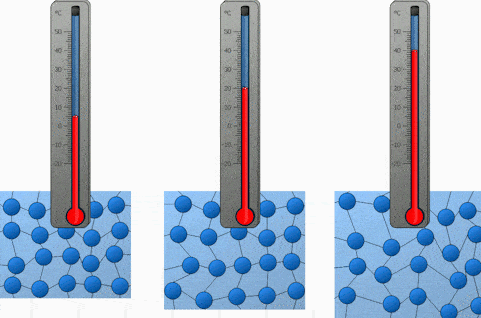
We will demonstrate the diffusion of food coloring in water and observe how it’s affected by the difference in temperature. Onwards to the experiment!
Materials needed for demonstrating Diffusion

- 2 transparent glasses – Common clear glasses will do the trick. You probably have more than needed around the house. We need one for warm water and one for cold water so we can observe the difference in diffusion.
- Hot and cold water – The bigger the difference in temperature in two glasses, the bigger difference in diffusion will be observed. You can heat the water to near boiling or boiling state and use it as hot water. Use regular water from the pipe as “cold water”. That is enough difference to observe the effects of temperature on diffusion.
- Food coloring – Regular food coloring or some other colors like tempera (poster paint) will do the trick. Color is required to observe the diffusion in our solvent (water). To make it more fun, you can use 2 different colors. Like red for hot and blue for cold.
Instructions for demonstrating diffusion
We have a video on how to demonstrate diffusion at the start of the article so you can check it out if you prefer a video guide more. Or continue reading instructions below if you prefer step by step text guide.
- Take 2 transparent glasses and fill them with the water. In one glass, pour the cold water and in the other hot water. As we mentioned, near-boiling water for hot and regular temperature water from the pipe will be good to demonstrate the diffusion.
- Drop a few drops of food coloring in each cup. 3-4 drops are enough and you should not put too much food color. If you put too much, the concentration of food color will be too large and it will defuse too fast in both glasses.
- Watch closely how the color spreads. You will notice how color diffuses faster in hot water. It will take longer to diffuse if there is more water, less food color and if the water is cooler.
What will you develop and learn
- What is diffusion and how it relates to osmosis
- Factors that influence diffusion
- What is Brownian motion
- How to conduct a science experiment
- That science is fun! 😊
If you liked this activity and are interested in more simple fun experiments, we recommend exploring all about the heat conduction. For more cool visuals made by chemistry, check out Lava lamp and Milk polarity experiment. And if you, like us, find the water fascinating, definitely read our article about many interesting properties of water.
If you’re searching for some great STEM Activities for Kids and Child development tips, you’re in the right place! Check the Categories below to find the right activity for you.

STEM Science
Videos, guides and explanations about STEM Science in a step-by-step way with materials you probably already have at your home. Find new Science ideas.
Read more
STEM Technology
Videos, guides and explanations about STEM Technology in a step-by-step way with materials you probably already have at your home. Find new Technology ideas.
Read more
STEM Engineering
Videos, guides and explanations about STEM Engineering in a step-by-step way with materials you probably already have at your home. New Engineering ideas!
Read more
STEM Math
Videos, guides and explanations about STEM Math in a step-by-step way with materials you probably already have at your home. Find new Mathematics ideas.
Read more
Psychology
Find out all about development psychology topics that you always wanted to know. Here are articles from child psychology and development psychology overall.
Read more
First year of Child’s Life
Following a Child’s development every month from its birth. Personal experiences and tips on how to cope with challenges that you will face in parenting.
Read more
4 thoughts on “How to Demonstrate Diffusion with Hot and Cold Water”